- +1 (212)-695-1962
- info@elementskit.com
- 463 7th Ave, NY 10018, USA
Secure your manufacturing license for Class A & B medical devices in India with Medfins' expert guidance and streamlined support, ensuring full CDSCO compliance
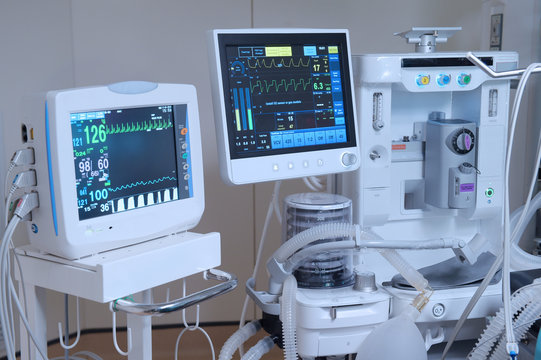
Processing time for MD 5 is 4-5 months.

License is issued by the State Licensing Authority (SLA).
Requires compliance with ISO 13485:2016 quality standards.
Break down your offerings as a list or grid:
Visual timeline or steps:
| Applicant Type | Application | License |
|---|---|---|
| Manufacturer | Form MD-3 | Form MD-5 |
| Test License | Form MD-12 | Form MD-13 |
| Applicant Type | Application | License |
|---|---|---|
| Manufacturer | Form MD-3 | Form MD-5 |
| Test License | Form MD-12 | Form MD-13 |
The fee as specified in Second Schedule of MDR-2017 is payable to the State Licensing Authority, through a challan or by electronic mode as may be specified by the State Government concerned.
Get the care you deserve